Cholesterol/Lipids
By: Mohamed Hamed MD, Eric Lieberman MD, Jonathan Vafai MD, Jonathan Kahan MD
What you need to know:
Lipids include both cholesterol and triglycerides, which are molecules of fat necessary for life. However, when our lipids are too high, they contribute to plaque development in the arteries of our body, which is called atherosclerosis. This process leads to most cardiovascular diseases.
This process is slow, with the buildup of plaque beginning in childhood and progressing over decades. There needs to be a combination of cholesterol (“wood”) and inflammation (“sparks”) from the immune system in order to create unstable plaques that rupture (“fire”), leading to heart attacks and strokes.
The standard lipid profile blood test has many pitfalls and moving to testing for the protein carriers of lipids, such as Apolipoproteins B and E, as well as the most atherogenic lipoprotein “little” a, are beneficial and much more relevant in terms of cardiovascular outcomes.
While smoking, diet, and lifestyle can have effects on cholesterol, there is a larger genetic component here than other sections of the book. Hints to a genetic cause are seen in the lipid profile and can then be confirmed by genetic analysis. Because of this, pharmacotherapy here is more important as patients may not be able to change their lipid profiles significantly.
What are lipids? What is cholesterol? What are triglycerides?
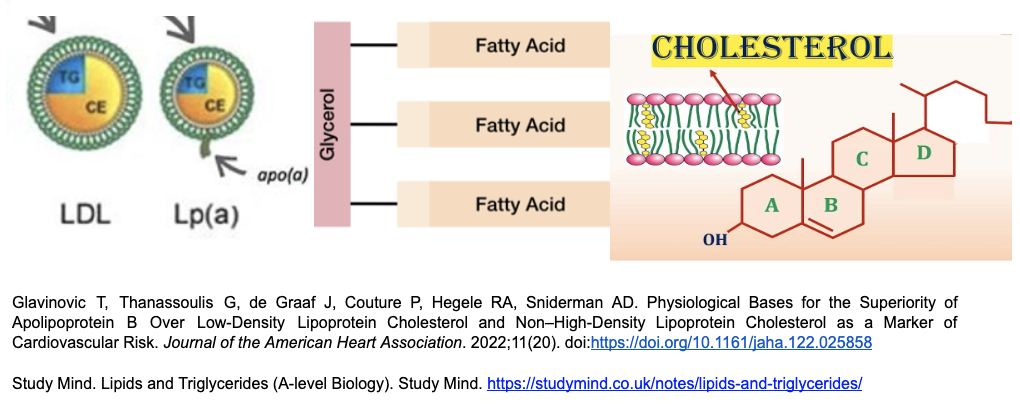
Lipids include both cholesterol and triglyceride and are non-soluble (does not dissolve in water) molecules that are transported by lipoproteins (proteins which are soluble and therefore can be carried by blood which is mainly water) 1,2,3. (See Figure 1)
Cholesterol is broken down into high density lipoproteins (HDL), low density lipoproteins (LDL) and very low density lipoproteins (VLDL). (See Figure 1)
Triglycerides (TG) are made up of three (3) fatty acid molecules attached to a glycerol backbone. These fatty acids are further classified into monounsaturated (aka omega 9), polyunsaturated (omega 3,6), saturated, or trans fats. Triglycerides (TG) outweigh cholesterol 10-100x in animal products. Plants do not contain cholesterol but have triglycerides 4,5. The TG measured in a blood test is an aggregate of all the TG that you ingest, as well as other sources such as the liver. (see figure 1 for structure).
Lipids are essential for your health, but too much of certain kinds of cholesterol/triglycerides can increase the risk of cardiovascular disease (this is called hyperlipidemia). Note that there really isn’t “bad” and “good” cholesterol as you will see below.
What does cholesterol do in the body? What do triglycerides do in the body?
Cholesterol is used for cell structure and function (cell membrane formation), in energy production, steroid hormones synthesis, bile acid production, and storage and transport of fat-soluble vitamins (Vitamins A, D, E and K) 6,7. Note oxidized cholesterol particles are highly pro-inflammatory and even certain non-oxidized particles can lead to inflammatory activation.
Triglycerides are used mainly for energy production and storage, as well as insulation in the form of adipose/fat cells and cell membranes 8.
What is a lipoprotein?
Lipoproteins are complex molecular structures composed of fats and proteins. They are responsible for transporting lipids to various organs. Think of a lipoprotein as a spherical cage that holds within it a liquid core of lipids. They are necessary because lipids are insoluble (think oil in water) and so they transport lipids around the body 9,10. We are concerned with 4 of them:
Apolipoprotein A1: the spherical protein cage that contains HDL (high density lipids).
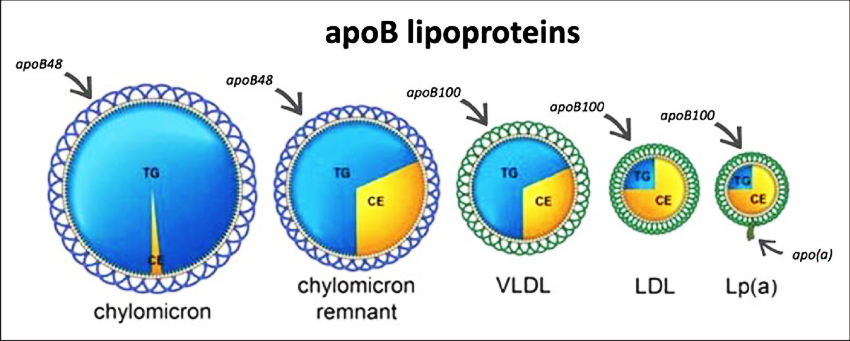
Apolipoprotein B (ApoB): the spherical protein cage that contains VLDL (very low-density lipids), IDL (intermediate density lipids) and LDL (low density lipids).
Lipoprotein “little” a (Lp(a)): the exact same as a small LDL/ApoB just with an extra protein tail.
Apolipoprotein E: the protein cage critical to transporting lipids and heavy metals across the blood brain barrier (both ways) and involved in neuron growth and repair.
Figure 1: Structures of 3 different molecules. The first on the left is an apoB protein holding a liquid center of cholesterols (CE) and triglycerides (TG). The liquid cholesterol center is like oil, which cannot dissolve in water. The apoB protein acts like a spiral cage which can dissolve in water (i.e. blood) and therefore move around the body, dropping off its liquid cargo at various sites. ApoB is the common carrier protein. The main difference between Lp(a) and ApoB is that it has lost most of its triglyceride cargo and has an extra tail. Triglycerides are 3 fatty acids connected to glycerol (middle pic). Cholesterol is structured as four linked hydrocarbon rings (right pic) 9-15.
How do we go from consuming cholesterol/triglycerides to plaque buildup in the arteries of our hearts/organs (aka atherosclerosis)?
A good analogy is this: cholesterol is the “wood” and inflammation is the “sparks” that create the fire of plaque rupture. Note inflammation is chronic here and comes from metabolic dysfunction +/- smoke/particle inhalation. You can skip the next section unless you want to do a deeper dive into cholesterol.
Can I get a “brief” summary on cholesterol transportation? (can skip if too complicated)
*****Note: See appendix for the full picture. It is very complicated, however understanding it will allow you to see how all the lipids relate to cardiovascular disease.******
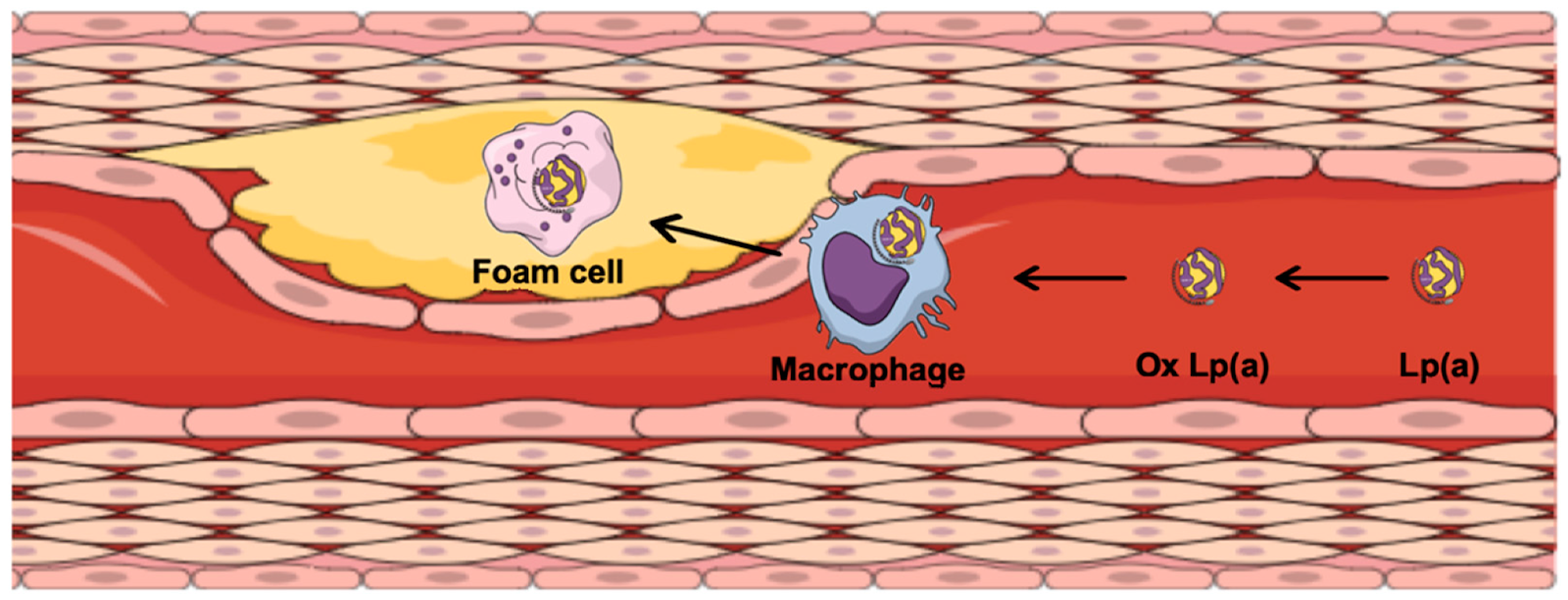
When we consume lipids (cholesterol and TG) they are broken down in our stomach and get absorbed into our bloodstream inside of a carrier called a chylomicron via the small intestines. The TG, LDL, VLDL (liquid cholesterols) get transported away from the liver to tissue inside the apoB cage, which can get stuck in the lining of the artery walls. Lp(a) is the smallest of these particles (the smaller the particle, the more likely it is to get stuck in the lining of the artery wall). The HDL particle brings cholesterols back to the liver16,17.
Once particles are stuck in the artery wall, they then become oxidized by free radicals, smoke, reactive oxygen species etc which causes immune cells to attack them (creating foam cells), leading to inflammation and plaque formation (aka atherosclerosis). Fat cells/metabolic dysfunction creates cytokines (messenger molecules) that can speed up this process. (see figure below)
Plaques begins as a small liquid center with a thin cap (see figure below), also known as a “vulnerable plaque”. When this cap breaks, the liquid, inflammatory center is exposed to the bloodstream where the body attacks it, and it becomes much larger very rapidly. This plaque rupture happens repeatedly and dynamically until 100% blockage is created. This is how heart attacks, strokes, congestive heart failure and death from cardiovascular disease occur. It is usually a sudden process at the end, however it starts when we are approximately 8 years old! 16-20
Note: See obesity chapter for how cholesterol/TG dropped off in muscle and other organs leads to metabolic dysfunction.
Ugovšek S, Šebeštjen M. Lipoprotein(a)—The Crossroads of Atherosclerosis, Atherothrombosis and Inflammation. Biomolecules. 2022; 12(1):26. https://doi.org/10.3390/biom12010026
Where does Cholesterol come from? Where do triglycerides come from?
Endogenous: Cholesterol and triglycerides are produced within the body (primarily the liver) and are highly affected by genetics and environmental factors. Note 70-80% of cholesterol comes from endogenous production 21,22. This is why medications can help significantly.
Dietary: comes through consumption of foods, especially animal-derived foods. The main sources are meat, eggs, cheese, and dairy products. The relationship between ingestion of dietary cholesterol and cholesterol in blood is weak at best. This is because eating cholesterol raises the large, buoyant LDL, which has less of an impact on plaque formation (the smaller the particle, the easier it is to get stuck in the artery walls). Note eggs have 200 mg of cholesterol each and is what is studied most (because they are easy to count and standardize). For example, the PURE trial showed that higher egg intake [>7 eggs/week vs, none] was not associated with any changes in cholesterol/blood lipids or any CVD outcomes. Similar results were found in ONTARGET and TRANSCEND trails (taken together >175,000 patients). Importantly, while the CHNS trial showed a decrease in all-cause mortality from eggs; red meat, pork, cheese and butter did not have the same results, which may relate more to other properties in eggs vs. meat. 21,23-26.
a. Saturated fat and excess carbohydrates, specifically added sugars like glucose and fructose, lead to preferential creation of triglycerides in the liver, which is sent to muscle cells and fat cells causing dysfunction (see obesity chapter) and that a low sugar diet can lead to decreases in triglyceride levels. Omega 3s can also lower TG, see omega section of this chapter.
What are the different types of fatty acids?
Remember that a triglyceride is just 3 fatty acids attached to a glycerol backbone. Saturation refers to the number of double bonds present. They can be in any combination of the fatty acids below:
Monounsaturated fats (MUFAs): Monounsaturated fats (olive oil, canola oil, peanut oil) have one double bond between carbon atoms and stay liquid at room temperature. These are also called Omega-9s (oleic acid is the common omega-9 in olive oil and 70% of the fats in avocado). Consuming monounsaturated fats increases HDL cholesterol and lowers LDL cholesterol.
Polyunsaturated fats (PUFAs): Have more than one double bond and stay liquid at room temperature (soybean oil, corn oil, nuts, seeds, fatty fish). PUFAs are broken down into Omega-3s (fatty fish, walnuts, chia, flax) and Omega-6s (soy, corn, sunflower, safflower oils). Note: these types of triglycerides are most vulnerable to oxidation outside the body (think rancid fish oil), leading to all the problems of oxidized lipids that are stuck in arteries of the body. Also note omega-6 go straight to the liver and can lead to fatty liver disease.
Trans fat: also unsaturated but have a different configuration making them solid at room temperature (think margarine, puff pastry, olive oil when it's burnt). Trans fats raise LDL levels, create reactive oxygen species, promote inflammation and plaque formation. These fats have largely been banned in North America.
Saturated fats: which have no double bonds between molecules and stay solid at room temperature (think butter, cheese, red meats). Consuming saturated fats does raise large, buoyant low -density lipoprotein (LDL) cholesterol by increasing apolipoprotein B production and decreasing the LDL receptor activity in the liver. However, consuming saturated fats also raises high density lipoprotein (HDL) and therefore the total cholesterol to HDL ratio (a marker for CVD) remains the same. Furthermore, the large buoyant type is less associated with plaque formation, unlike added sugars such as fructose which lead to small dense LDLs that are highly associated with plaque formation. Note eating a high saturated fat diet has not translated to worse CVD outcomes in large studies such as the Minnesota Heart Study when compared to vegetable oil. Note also that saturated fat does preferentially lead to fatty liver disease (outside the scope of this book, more so than fructose!). 27-30
****Important****: Different oils have different “smoke points” where the oil converts to trans fat and other harmful substances. Olive oil has a low smoke point (325°F) where heating it converts to trans fat. The oil with the highest smoke point is avocado oil (500°F). Olive oil is excellent for drizzling on foods but very poor for cooking because of this.
What evidence is there that elevated cholesterol and triglycerides lead to CVD?
There are multiple large trials that have shown that elevated cholesterol and triglycerides lead to CVD. On the cholesterol side the Framingham Heart Study, (one of the most famous cardiovascular studies) showed strong correlation between LDL cholesterol and CVD. Similarly, the Copenhagen City Heart Study showed fasting TG were independently associated with risk of heart attacks and ischemic heart disease, independent of age, smoking etc. 31-33
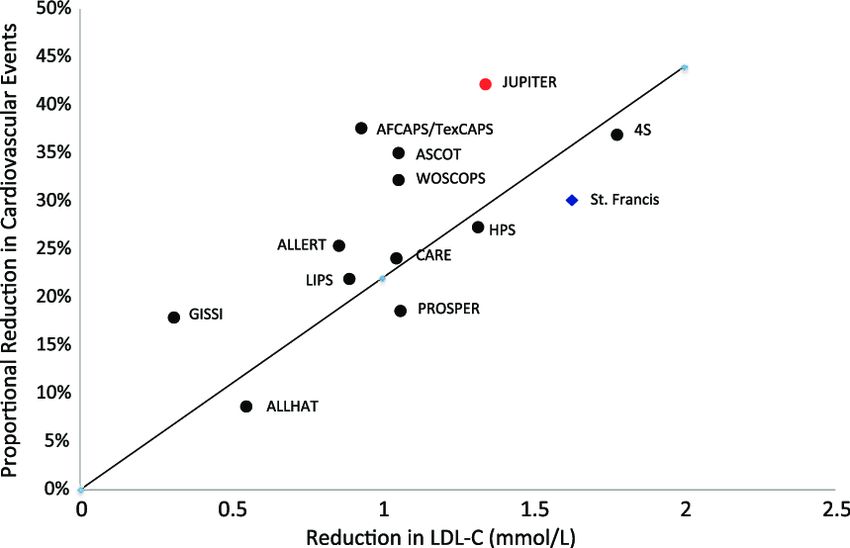
Relationship between Cardiovascular Events and Cholesterol Lowering Medications in the largest cardiac trials ever done on the subject. Over millions of patient-years there is a direct linear relationship between cholesterol reduction and reductions in cardiovascular events99.
How do we measure lipids?
The most common method used to measure lipid levels is the lipid profile.
What is lipid profile?
The lipid profile includes total cholesterol, high-density lipoprotein (HDL)-cholesterol, triglycerides (TGs) and low-density lipoprotein (LDL)-cholesterol (LDL-C). It is used as an important method for the risk assessment for cardiovascular disease.
VLDL and LDL-C have traditionally been calculated using the Friedewald equation, not through direct measurement. VLDL is calculated by TG/5 and LDL-c is calculated by Total cholesterol - HDL - VLDL 34.
What are the Pitfalls of these calculations?
This equation is not valid when the Triglyceride level is ≥400 mg/dL or <100 mg/dL. Also, TG varies with state of fasting, recent meals, recent alcohol use and metabolic conditions such as diabetes. You can measure LDL-c directly with an additional blood test order. Studies suggest repeating the LDL-c by direct assay techniques in patients with triglyceride >200 mg/dL or when LDL<70 or >130 mg/dL 34-36.
Are there limitations for direct LDL-C assays?
There are no standard methods to directly measure LDL-c. There are multiple assays to measure LDL produced by different manufacturers, and different labs use different assays which can show inaccurate results.
It is more expensive for direct LDL measurement as it needs additional reagents and labor compared with the calculated LDL-C method. You may have also heard about measuring the different densities of LDL, with small dense LDL being more atherogenic. However, the same exact problems persist, in that different labs use different magnets to measure the density of LDL, and there are no standards. Furthermore, it doesn’t include the most atherogenic particle which is Lp(a) 36-38.
What is Non-HDL Cholesterol?
This is measured by taking the total cholesterol and subtracting HDL cholesterol levels. This accounts for LDL-C, VLDL-c, triglycerides, and cholesterol remnant levels, all particles considered to be atherogenic. This measurement may be a better estimate of “bad cholesterol” than just the LDL-c alone.
What is the most accurate way of measuring “bad cholesterol” and cardiac risk?
Apolipoprotein B (Apo B) is the protein cage enclosing all VLDL, IDL, LDL particles and Lp(a) can be measured directly. These labs are unrelated to the fasting state of the patient and are uniform among laboratories. Elevated levels of apo B are associated with increased risk of cardiovascular disease. There is substantial evidence that apoB levels measure the atherogenic risk better than LDL or non-HDL cholesterol. In addition, only ApoB particles [and Lp(a)] can enter the artery wall and create atherosclerosis 39-41.
Glavinovic T, Thanassoulis G, de Graaf J, Couture P, Hegele RA, Sniderman AD. Physiological Bases for the Superiority of Apolipoprotein B Over Low‐Density Lipoprotein Cholesterol and Non–High‐Density Lipoprotein Cholesterol as a Marker of Cardiovascular Risk. Journal of the American Heart Association. 2022;11(20). doi:https://doi.org/10.1161/jaha.122.025858
Approximately half of all patients with recurrent heart attacks have normal cholesterol levels on standard lipid profiles, and despite having achieved the recommended LDL, these patients are still at high risk of cardiovascular-related events. Because there is a 1:1 relationship between an apoB molecule and an LDL core, it always correlates to risk of CVD. If there are numerous apoB molecules but each one carries normal LDL amounts, then the LDL measurement in blood will be normal, despite elevated risk. Conversely, large LDL cores can lead to falsely elevated LDL levels, which exaggerate risk, despite normal apoB levels. This occurs in 7% of diabetic patients and 15% of patients with metabolic syndrome have normal apoB with elevated LDL. These patients do not develop cardiovascular disease and do not need cholesterol lowering therapy 39-43. Apo B level more than 130 mg/dl is considered elevated risk for the general population and < 90 mg/dL for any high risk population for cardiovascular disease (see below) 44.
What elevates ApoB?
Diet: Saturated fats and trans fats can increase the production and secretion of apoB-containing lipoproteins.
Obesity: Visceral fat (see obesity section) can secrete various hormones and cytokines that influence lipid metabolism and increase the levels of apoB-containing lipoproteins.
Physical inactivity: sedentary lifestyle and lack of regular exercise. Exercise increases metabolic demand from muscles, increasing free fatty acid and TG use. Additionally, it raises HDL levels (think revving up the system), enhances LDL receptor activity in the liver to suck back cholesterol particles and reduces the metabolically active visceral fat.
Genetics: genetic factors which include mutations in genes involved in lipid metabolism. For example: familial hypercholesterolemia. Genetic factors are prominent in lipid disorders. Again, this is the one area where pharmacology will play a more prominent role.
Insulin resistance: including conditions like metabolic syndrome and diabetes mellitus.
Liver diseases: The liver is the most important organ in lipid metabolism and impaired liver function can disrupt lipid metabolism leading to elevated levels of apoB.
Smoking: Tobacco use has been associated with dyslipidemia and increased levels of apoB-containing lipoproteins. 39-43
What is Lipoprotein(a) [Lp (a) aka Lp “little” a]
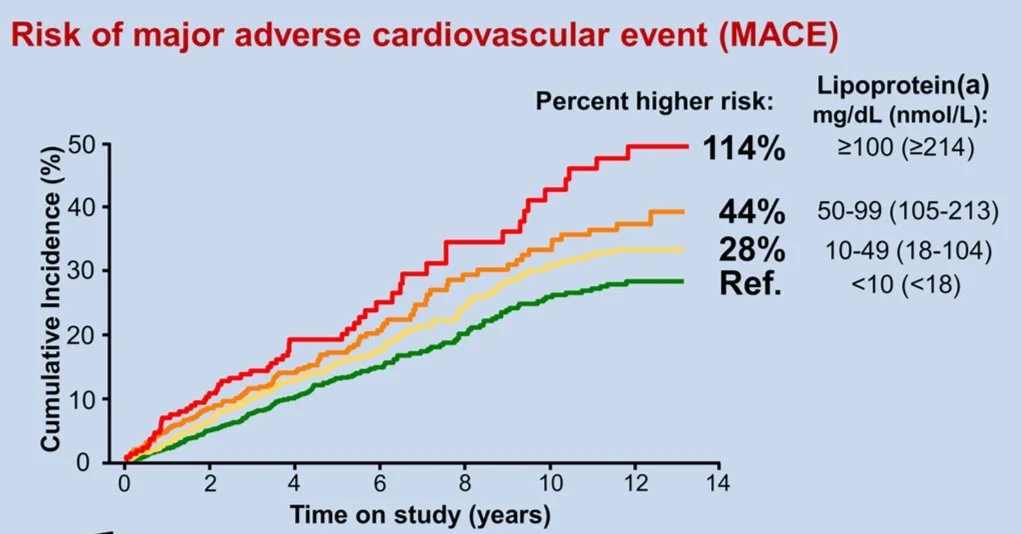
Lp (a) is a type of lipoprotein particle composed of LDL cholesterol (in an apoB) and bound to apolipoprotein(a) (the tail). It can induce vascular inflammation, atherogenesis, calcification and thrombosis because it is the smallest cholesterol particle and contains actual oxidized phospholipids on it (oxidization of phospholipids is how plaque is formed in the arteries). Lp (a) levels are heavily determined by genetics. Patients with family history of elevated Lp(a) are at increased risk for cardiovascular disease and it may even cause aortic valve narrowing. It cannot be influenced by lifestyle factors like diet and exercise in the same way other particles are 38,45-47. The optimal Lp(a) levels are less than 50 mg/dL 46.
The higher the level of Lp(a) the higher the risk of MACE (major adverse cardiac event ie. Heart attacks, strokes, heart failure)
****** Statins and ezetimibe (Zetia) do not effectively lower Lp(a), in fact they may paradoxically increase its concentrations up to 30%. PSCK-9 inhibitors can decrease the levels by 30-40%. There are specific drugs targeting Lp (a) in development 48-50.
What is Apolipoprotein E (Apo E)?
Apo E is a protein involved in lipid metabolism and transport, especially in the blood brain barrier (a series of blood vessels that surround the brain, controlling what gets in/out). There are 3 major variants: apoE2, apoE3 and apoE4. A Blood test can determine allele status (E2E2, E2E3, E2E4, E3E3, E3E4, and E4E4) 51-53.
What does ApoE do?
Cholesterol transport: It has a key role in the metabolism of lipoproteins and cholesterol transport. In addition, it has an important role in the removal of triglyceride-rich lipoproteins from the circulation.
Alzheimer’s disease: It has been implicated that apoE has a role in the pathogenesis of Alzheimer’s disease. Apo E has a role for clearance of heavy metals and beta amyloid protein from the brain, as well as maintenance of the blood brain barrier.
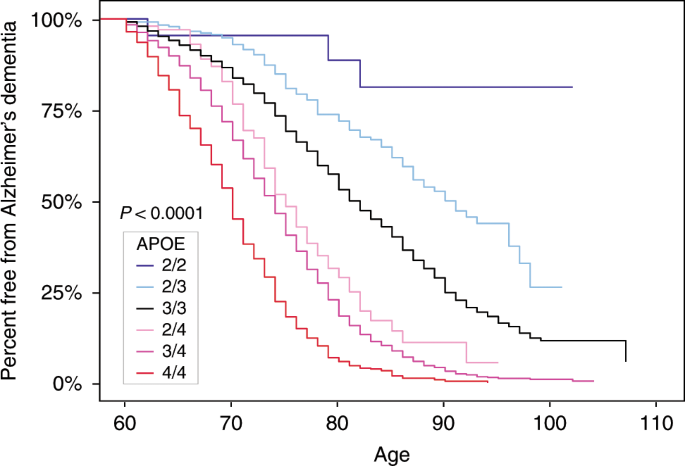
- Apo E2 allele provides protection against Alzheimer’s.
- Apo E3 is the most common allele and has neural effect on the disease
- Apo E4 allele is a major genetic risk factor for the onset of Alzheimer’s disease. Carriers of apo E4 allele have a higher risk for developing Alzheimer’s disease with earlier age of onset. Around 15-25% of people have at least one allele copy 51-54.
APO-E and likelihood for development of Alzheimer’s dementia
Reiman EM, Arboleda-Velasquez JF, Quiroz YT, et al. Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nature Communications. 2020;11(1).doi:https://doi.org/10.1038/s41467-019-14279-8
The apo E4 allele responds significantly to a low saturated fat diet (more so than pharmacologic intervention), resulting in lower apoB and triglycerides and a potential decreased risk of developing Alzheimer’s dementia (evidence limited for this). It is controversial whether omega-3 has an effect. Overall, we recommend the aggressive lifestyle, diet and pharmacologic interventions in this book if you are diagnosed with an APOE-4 allele 54.
Wait, so I can inherit cholesterol issues?
Yes! There are a multitude of common inherited hyperlipidemia disorders called familial hyperlipidemias. We suspect a patient may have these if they have a personal or family history of early cardiovascular disease (defined as a CVD event in men before the age of 55 and women before the age of 65), abnormally high lipid levels despite diet and exercise (especially in children), and certain physical characteristics. Classically, familial hyperlipidemia with isolated high LDL-c responds extremely well to a low saturated fat diet (affects 1 in 250 people). Familial Hypertriglyceridemia may respond to omega 3 fatty acids or other lipid lowering therapies. The most common is familial combined hyperlipidemia which affects 1 in 50 people! It is one of the few cholesterol conditions that responds well to a combined low saturated fat and high omega 3 diets. The rest will require some combination of lifestyle and cholesterol lowering medication (see below). This class of cholesterol issues belies the importance of checking lipid profiles early! 55-57
So what diet should I go on to lower my cholesterol? I don’t need medication right?
Diets low in saturated fat (and/or high in polyunsaturated fat) lead to reduced liver cholesterol production and upregulation of the LDL receptors, leading to increased cholesterol clearance. Excess calories from simple carbs and saturated fat lead to increased VLDL production. Regular exercise improves insulin sensitivity, resulting in less VLDL production. However, in randomized control trials and meta-analysis, intensive dietary changes (reducing saturated fat to <10% of diet for example) lead to only a 5-10% reduction in apoB and a 0%-5% reduction in lp(a)100,101.
Ok ok, so what are the general goals of treatment for lipids?
Guidelines vary, however the generic answer is the lower the better. We have not found a patient who has “too low” cholesterol. Infants have a total cholesterol of around 60 mg/dL to put things in perspective. Also remember that plaques can begin to form when a person is around 8 years old, so the younger the better to start treatment also is vital 20,58. We recommend optimal levels of fasting triglycerides of <150 mg/dL, apolipoprotein B levels of < 80 mg/dL and Lp(a) of < 50 mg/dL64-66. Note we do not recommend LDL goal levels given the issues listed above. These reflect the high risk group goals which were poorly defined or not defined across multiple studies, airing on the side of caution given the great treatments currently available to achieve those goals.
Who currently gets treatment for Cholesterol?
The most commonly used guidelines are the US Preventive Services Task Force (USPTF) guidelines.
Current USPTF guidelines:
Adults who are age 40-75 with at least one CVD risk factor (smoking, HTN etc)
ASCVD risk 7.5% (consider prevention), >10% recommend prevention
Diabetics
LDL-c > 190 mg/dL
Note: Currently it is not recommended to start statin therapy in patients aged 76 or older for primary prevention by the USPTF 59,60
What is ASCVD?
ASCVD (Atherosclerotic cardiovascular disease) is a risk calculator using various parameters to gauge a person’s 10-year risk of having a heart attack or stroke 61. It is used heavily for determining a patient's 10-year cardiovascular risk. However, several problems come up which in our opinion limits its use.
The ASCVD: What’s missing?
Heavily dependent on age
It is probably too late (Atherosclerosis process starts early!)
ASCVD not explained by major risk factors
No family history (especially family history of premature ASCVD)
It does not incorporate calcium score data
No other cholesterol factors other than lipid panel (Lp(a) levels ≥125 nmol/L (≥50 mg/dL) are considered an ASCVD risk-enhancing factor)
Atherosclerosis starts during youth; the earliest lesions can be identified in the arterial beds of most adolescents. Rapid progression of these early lesions occurs in the 3rd and 4th decades of life. This suggests that the optimal age to begin the prevention of atherosclerosis is as young as possible. Earlier studies have suggested that the age at this effect becomes statistically significant is 8 years old. It is still unknown who would most benefit from more aggressive, pharmacological intervention to lower risk 20.
What are the current recommendations from other guidelines?
For low-density lipoprotein (LDL) cholesterol levels in patients with diabetes, the ADA now recommends a target of less than 70 mg/dL or no greater than 55 mg/dL, depending on the individual’s cardiovascular risk (note apoB is not factored in) 62. For people with diabetes aged 40-75 years at increased cardiovascular risk, including those with one or more atherosclerotic risk factors, high-intensity statin therapy is recommended to reduce LDL cholesterol by 50% or more from baseline and to a target of less than 70 mg/dL 63. Also targeting an Lp(a) of < 50 mg/dL is important 64. Note that risk here should include a number of factors including family history, smoking status, metabolic status, kidney/blood pressure measurements, lipid levels, activity level, race, and diet. In general, the lower the cholesterol and lipids, the better.
Is there a new calculator by the American Heart Association?
There is a new calculator that starts screening at an earlier age which is important, however similar problems persist. It is still dependent on age more than any other factor, does not start early enough, has no family history, and does not include a coronary calcium score and is heavily reliant on cholesterol levels with no accounting for Lp (a) or Apo B. It also removes race, which was an important consideration for individual risk67.
What is a coronary artery calcium (CAC) scoring (CT calcium scoring)?
This is a non-invasive imaging technique used to assess calcium in the arteries that supply blood to the heart (coronary arteries) by CT scanner (no IV, no contrast used). Coronary artery calcium is a marker of atherosclerosis (narrowing of the arteries supplying blood to the heart). CAC can be used to assess risk of developing coronary artery disease which can lead to cardiovascular events including heart attacks. Remember from the cholesterol model that mild vulnerable plaque (20% blockage etc) can rupture in an instant and become severe (eg. 80% blockage). This is why routine stress testing is unhelpful as it only picks up plaque that is already advanced 68,69.
Who should get a CAC?
Anyone above the age of 40 who does not already have coronary artery disease. It is especially helpful for those at intermediate risk (10-20% on 10 year risk calculators). This can be repeated every 3 years 70. Please understand that by definition, an elevated calcium score indicates that you have coronary artery disease (CAD) aka plaque in the arteries of your heart. While this book is geared towards prevention of disease (ie. primary prevention), being diagnosed with CAD means that you already have the disease. However, the topics in this book also apply to secondary prevention (preventing a disease once it is already present) and are still recommended.
What do the calcium scores mean?
Low Risk (Score: 0-100):
Score 0: No detectable coronary artery calcification. Generally associated with a very low risk of coronary events in the next 5-10 years, well below 1%.
Score 1-100: Mild calcification. Suggests a low risk of coronary events, but slightly higher than with a score of 0. The risk of coronary events ranges between 1% to 10% over the next decade, depending on the exact score and patient-specific factors like age and sex.
Follow what's in this book, borderline need medications (discuss with your physician).
Medium Risk (Score: 101-400):
Moderate calcification. Indicates a more significant presence of coronary artery disease. Patients with scores in this range typically have a 10-20% risk of coronary events within the next 10 years.
Follow what's in this book, plus possible need for medication (discuss with your physician).
High Risk (Score: >400):
Extensive calcification. Indicates a high likelihood of at least one significant coronary artery obstruction. Patients with scores above 400 are at a high risk of coronary events, with more than a 20% risk of experiencing a coronary event within 10 years.
Follow what’s in this book, high likelihood of needing cholesterol medication (discuss with your physician).
Calcium scoring prognosis:
van Werkhoven JM, Bax JJ, Nucifora G, et al. The value of multi-slice-computed tomography coronary angiography for risk stratification. Journal of Nuclear Cardiology: Official Publication of the American Society of Nuclear Cardiology. 2009;16(6):970-980. doi:https://doi.org/10.1007/s12350-009-9144-3
Should women get a CAC?
Women are underrepresented in prior risk scores. CAC can help detect early signs of atherosclerosis in women who may not otherwise be identified as high risk. CACs are an excellent screening tool and utilize very low dose radiation compared to other stress testing.
What are the limitations of the CAC?
Studies showed that the prevalence of noncalcified plaque was 11.1% in patients with no CAC and 23.4% in the mild CAC group. Multiple plaques were detected in 2.6% of the group with no CAC and 3.7% of the group with mild CAC. Significant coronary artery stenosis was found in one patient in the group with no CAC (0.9%) and three patients in the group with mild CAC 71.
What is a CT coronary angiogram?
It is a non-invasive imaging technique that uses intravenous contrast to visualize the coronary arteries. It gives detailed images of the arteries which can detect narrowing, blockages and other abnormalities of the coronary arteries. It can also differentiate between stable and unstable plaques. Unstable plaques have higher risk for acute coronary events.
CT coronary angiography is the only imaging test which has shown a reduction in CVD mortality and myocardial infarction as demonstrated by SCOT-HEART trial. This is because it allows more preventive therapies to be initiated earlier, in addition to more antianginal therapies72.
Ok so how do I actually treat high cholesterol and high triglycerides? (Hint: read the rest of the book!)
Diet (see obesity section)
Exercise (see exercise section)
Weight loss (see obesity/exercise section)
Smoking cessation (see smoking section)
Limit Alcohol (see obesity section)
Medications
Although there are a lot of treatment options, it is still largely determined by genetics except high triglycerides which can be much more affected by diet, exercise, limiting alcohol/added sugars. Medications include the statins, Ezetimibe (Zetia), the PSK9 inhibitors, and Bempedoic Acid.
I’ve heard of Statins! I’ve also heard bad things about them?
Statins are the most famous class of medications used to lower cholesterol levels, especially LDL-c. They can also modestly increase HDL-c levels and reduce triglycerides. Statins have been used in patients with established coronary artery disease, and for those with a high risk of cardiovascular events.
Statin therapy reduced the risk of major coronary events, major cerebrovascular events, and revascularizations by 29.2%, 14.4%, and 30%, respectively. Statins produced a nonsignificant reduction in coronary heart disease mortality (22.6%) and overall mortality. There were statistically significant permanent increases in cancer or liver failure 73,74.
The Number Needed to Treat (NNT) to prevent one major vascular event in those at the lowest levels of risk for which statins could be recommended was 40 according to the 1994 and 1998 guidelines (i.e. an ASCVD of 20%); 73 according to the 2004 and 2007 guidelines; and 400 according to the 2012 and 2016 guidelines (i.e. an ASCVD of 7.5%). This means that by today's standards, you would need to treat about 268 people over 5 years to prevent one stroke, and approximately 60 people to prevent one non-fatal heart attack 74,75.
Ok but what about side effects?
Statins are generally well tolerated. Most common side effects are muscle aches (10%) and elevated liver enzymes. It rarely can cause muscle damage called rhabdomyolysis. Statins can cause muscle aches as it inhibits an enzyme in the liver which is also located in the muscles 76. Coenzyme Q 10 has been used to prevent muscle aches associated with statins (although there is no evidence that it works). When you have these side effects it is recommended to just switch to a different statin 77. Pravastatin works on a different enzyme in the liver and therefore is considered the mildest in terms of side effect profile 78.
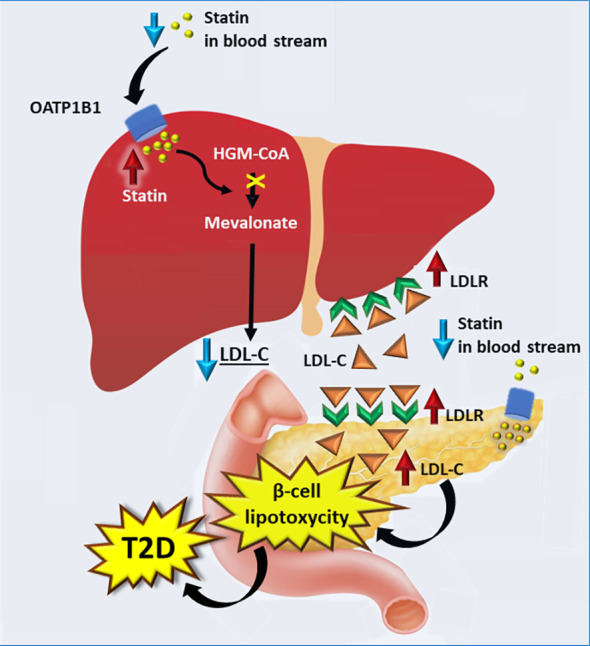
Do statins cause Diabetes/Insulin Resistance?
The mechanism for statins leading to diabetes is as follows: statins inhibit LDL production, which leads to an increase in LDL receptors on the liver but also the pancreas. This floods the pancreas with LDL which damages the beta cells that make insulin, leading to decreased insulin and potential risk of diabetes. Other theories include insulin resistance by FFA release from fat cells, or changes to the glucose transporters themselves.
However, in studies this effect is only seen at the highest doses of atorvastatin which is 80 mg. The Number Needed to Harm (ie. the number of patients on statins for 5 years to get one case of diabetes) is about the same as the NNT for a non-fatal MI (67). We recommend decreasing statins to the lowest level possible to achieve cholesterol guideline goals. Approximately 85% of that goal is achieved with rosuvastatin 10 mg or atorvastatin 20 mg in most patients.
Image: laakso M., Silva LF. Statins and risk of type 2 diabetes: mechanism and clinical implications. Front. Endocrinol. 18 Sept 2023, Volume 14-23.
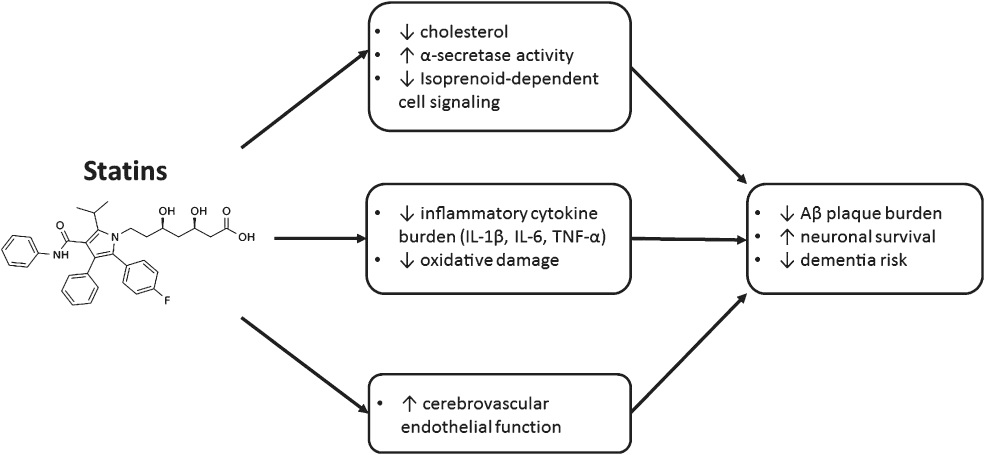
Do Statins cause Alzheimer’s dementia?
Overall Statins have a protective effect on Alzheimer’s disease as it decreases triglycerides. However, reducing triglycerides is not enough of an explanation. They have other benefits such as anti inflammatory properties and overall can lead to a reduction in dementia risk 81.
Padala, Kalpana & Potter, Jane & Ikezu, Tsuneya. (2009). HMGCoA-Reductase Inhibitors in Dementia: Benefit or Harm. Clinical Medicine : Geriatrics. 2009.
What about ezetimibe (Zetia)?
Ezetimibe is one of the medications that can lower cholesterol levels by blocking intestinal absorption of the cholesterol in the small intestine. It is usually used as an adjunctive therapy with statins if the target level is not achieved with statins or can be used as monotherapy in patients not tolerating statins 82.
*Overall, there is limited evidence of ezetimibe in primary prevention.
Previous study showed that ezetimibe has improvement in cardiovascular events but this study was done in Japan and included patients > 75 years
Ezetimibe reduced the incidence of the primary outcome of heart attacks by 34%. Regarding the secondary outcomes, the incidences of composite cardiac events of 40% and coronary revascularization were 62% lower in the ezetimibe group than in the control group; however, there was no difference in the incidence of stroke, all-cause mortality, or adverse events between trial groups. 83
What about Fibrates?
Fibrates help break down triglycerides and are mainly used for their reduction, as well as a potential increase in HDL-c, while having a limited effect on LDL-c/ApoB. There have been mixed results in large scale trials, and therefore these are typically not recommended as first line agents and are mainly used in patients with very elevated triglycerides (think familial hypertriglyceridemia) 84.
What about PCSK9 inhibitors, the injections?
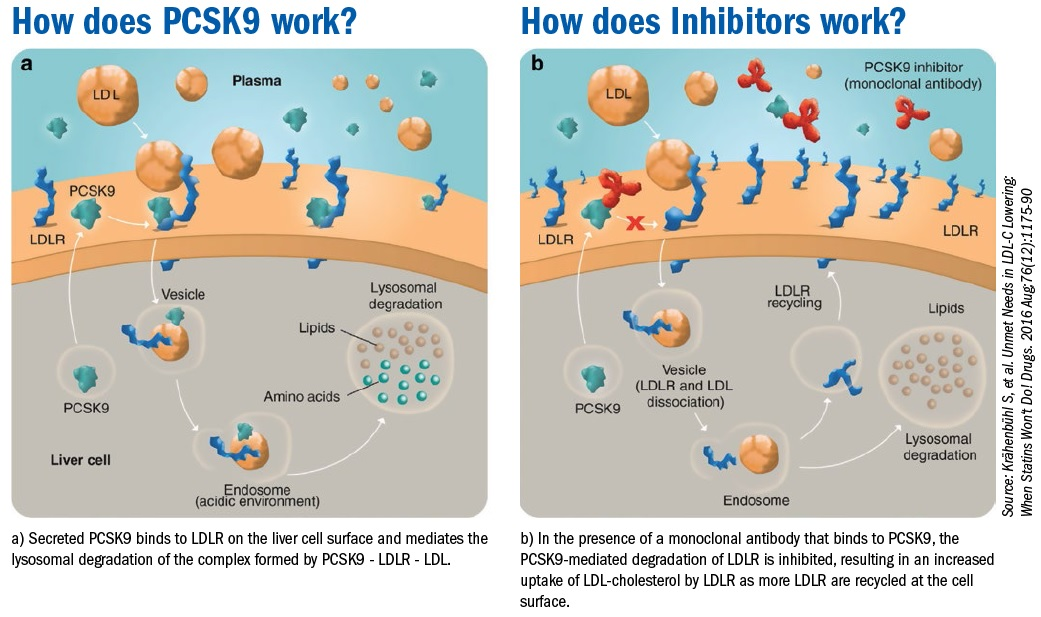
PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors are a class of medication that are used to treat high cholesterol, especially when other medications (statins or ezetimibe) were not effective or not tolerated. PCSK9 inhibitors include evolocumab, and alirocumab. PCSK9 inhibitors are potent inhibitors of LDL receptor degradation which help clear LDL cholesterol from the blood 85, 86.
They reduce the level of LDL in addition to a reduction of Lp (a) levels. PCSK9 inhibitors can reduce LDL levels up to 60-70% alone or when used with statins or other cholesterol lowering medications 87.
Reduction in the level of LDL-c by around 53% 87
Reduction of LDL-c by 39% when compared with ezetimibe (Zetia) combined with a statin 87
It has also shown a reduction of the risk of cardiovascular events when compared with placebo, and when compared with ezetimibe(Zetia) combined with a statin 88
PCSK9 inhibitors are particularly useful in familial hypercholesterolemia (a genetic condition characterized with very high levels of cholesterol that occurs from birth). In the FOURIER trial, Evolocumab showed reduced cardiovascular mortality, myocardial infarction, or urgent revascularization 89. They are the only known class of medications to significantly reduce Lp(a) by 30-40% (although targeted therapy will be coming out in the next couple of years for this condition) 90,91. In addition, Evolocumab may also reduce the progression of aortic stenosis and need for aortic valve replacement (possibly related to Lp(a)) 92. PCSK9 inhibitors are typically self-administered by subcutaneous injections that are usually given every 2-4 weeks, depending on the specific medication and dosage regimen.
Side effects?
None unless specifically allergic to antibody
What about bempedoic acid (Nexletol)?
It inhibits an enzyme that is involved in making LDL-c. It only acts in the liver so diabetes risk is significantly less. The CLEAR study showed that Bempedoic acid showed a mean reduction of LDL 29.2 mg/dL compared with placebo. It showed improvement of composite outcome including MACE, defined as cardiovascular death, nonfatal MI, not fatal stroke or coronary revascularization (11.7% vs 13.3%). Bempedoic acid did not show significant effect on stroke, death from cardiovascular causes or all-cause death 93.
Side effects: it increases the incidence of gout and gallstones.
Wait, you haven’t mentioned HDL-c once! Doesn’t having a high HDL negate any bad cholesterol?
We understand very little about HDL (“good cholesterol”) and the complex interactions these particles have in cholesterol transport. While HDL in theory brings cholesterol back to the liver and reduces plaque burden, efforts to raise HDL with medication called CETP inhibitors (an enzyme that when blocked raises HDL) have failed to improve outcomes. Moreover having a high HDL does not negate a high apob/LDL in terms of CVD outcomes. One CETP inhibitor, obicetrapib, one of the strongest ever developed, is showing promise, however there is no outcome data at this time 94.
What about Fish Oil and Omega-3 Fatty acids?
Omega-3s are an essential nutrient (not produced by our body) and therefore must be obtained from diet. A number of trials have shown that low levels of omega 3 can lead to dementia, anxiety, cognitive decline and cardiovascular events such as heart attacks and strokes. There are several omega-3 fatty acids including ALA (Alpha-Linolenic Acid), EPA (Eicosapentaenoic Acid) and DHA (Docosahexaenoic Acid). Broadly, EPA is used for cardiovascular disease prevention while DHA is used for brain health. ALA is the precursor to EPA and subsequently DHA (but conversion to EPA is more efficient (5-10% conversion vs. DHA which is 2-5%) 95,96.
ALA: found mainly in plants including flaxseed, chia seeds, walnut, and echium seed oils
EPA: found mainly in fish including salmon, mackerel, sardines, and anchovies.
DHA: found mainly in fish including salmon, tuna, sardines, shellfish and herring.
What do omega-3 fatty acids do?
Omega-3 can reduce inflammation, are important for brain function and vision, lower triglyceride levels (block TG formation from carbohydrates/improve TG clearance), reduce VLDL (“bad cholesterol”, blocks ApoB particle formation/VLDL production) and can help with reduction of blood pressure.
How do you measure your omega 3 levels?
You can measure it with a blood test or an at home test using a finger prick. The goal is to have levels to as high a dose as possible. The mean omega-3 index of dolphins is 19.9% percent compared with 9% for Japanese people and 5% for Americans 96.
What is the evidence?
Data is very mixed on the beneficial roles of fish oils in general. The GISSI-Prevenazione Trial in 1999 found that heart attack survivors who took omega 3 lowered their chances of having a fatal heart attack, and studies done in 2012 showed that DHA improved brain function in older adults and reduced inflammation. However, only two prior studies (JELIUS and REDUCE-IT) showed the benefit of the EPA only version of fish oil in the reduction of major adverse cardiovascular events and cardiovascular death.
Omega-3 administration could be associated with higher risk of gastrointestinal side effects and higher incidence of investigator reported atrial fibrillation (which causes strokes) and bleeding. A Cochrane review in 2018 (amalgamation of all the evidence) showed no benefits of supplemental fish oil on mortality or cardiovascular health (although most trials used much lower doses of fish oil than recommended below). Also, as stated above, fish oil is a PUFA (polyunsaturated fat) which is prone to oxidation (when rancid) which is how the process of plaque formation occurs. Flavored fish oils have a significantly higher chance of being rancid than unflavored. A common theme of the only two positive trials of fish oil was that they were EPA only (although DHA is posited to be helpful for brain health) and that the doses were very high (like 4 grams per day!).
Overall, we recommend, when possible, to obtain most of your omega 3 from actual fish sources, which has been shown to have benefit in a number of trials without the side effects 94,96-98. Note that “wild caught” fish have higher omega 3 levels than farm raised fish, which are fed corn instead of algae. Sardines are also an excellent source, and mercury levels can be checked with a simple blood test. If you need to supplement further (based on omega index levels), we recommend high dose (like 3-4 grams, EPA only fish oil, unflavored, from a third party tested and trusted source). If needed, rTG has better absorption than EE version. Lastly, be mindful if you take medications that can also cause bleeding or if you have conditions such as atrial fibrillation, that can be exacerbated by fish oils.
Key Points: Cholesterol Management
This is the most difficult section of the book, and is confusing even to clinicians. Cholesterol represents the “wood”, inflammation represents the “sparks” which make the “fire” of atherosclerosis aka plaque in the arteries of the body.
In general, check your lipid panel (for TG levels), apoB and Lp(a) as early as possible to accurately assess your lipid status. Repeat these at least annually. The goal is TG < 150 mg/dL, apoB < 80 mg/dL and Lp(a) < 50 mg/dL.
Imaging tests such as calcium scores and coronary CT scans are helpful to detect plaque which won’t show up on standard stress tests. This process starts when you are a child and builds up over decades.
Management of hyperlipidemia should include lifestyle modifications such as diet, exercise, weight loss, and smoking cessation, in addition to medications. Understand that a lot of this is genetically driven, and more than any other section there is a role for pharmacologic management.